Medical Device Development Services
Total support for entry of foreign medical devices into Japanese market
[Scope of Service]
- Class I, II, III, IV
[Major Services]
- Design of effective pharmaceutical affairs strategies for obtaining license for marketing authorization holder, license for manufacturing, and accreditation of foreign, etc.
- Support for the application of Approval / Certification / Registration
- Consulting, preparation of documents for application, corresponding to PMDA or third party certification body, feasibility study of foreign clinical data and others.
- GCP Audit of overseas/local clinical trial data and preparation of audit report (English available)
- Operation of local clinical trial and post-marketing surveillance.
- Preparation of clinical evaluation report
- Clinician and co-medical interview
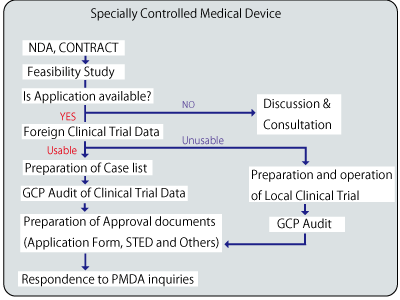
Example: Our Support Process for Application of foreign Medical Device
Our priority is to actively use the Foreign Clinical Trial Data for cost reduction.